Design Controls in Device Development
Understanding the needs of your user and constraints to guide ideation and quality device design. See an overview from the FDA here
ASME
The American Society of Mechanical Engineers. Wide range of standards covering everything from power plants to simple mechanics.
ASTM International
American Society for Testing and Materials. 12,800+ technical standards covering materials to services.
ISO
International Organization for Standardization. Standards covering quality, health and food safety and more.
GT Library Standards
GT library provides access to ASTM International, IEEE, SAE, ASCE, and more.
IHS Markit
Source for hard copy standards in a range of engineering topics and repository from multiple standard organizations.
IEC 60601
Standard from ISO regarding medical equipment frequently used by students.
NASA Workmanship
Companion to the NASA Technical Standard, NASA-STD-8739 series of workmanship requirements documents and regards electrical and electronic equipment acceptance/rejection standards.
Generating New Ideas. Solving Big Problems
FDA Resources
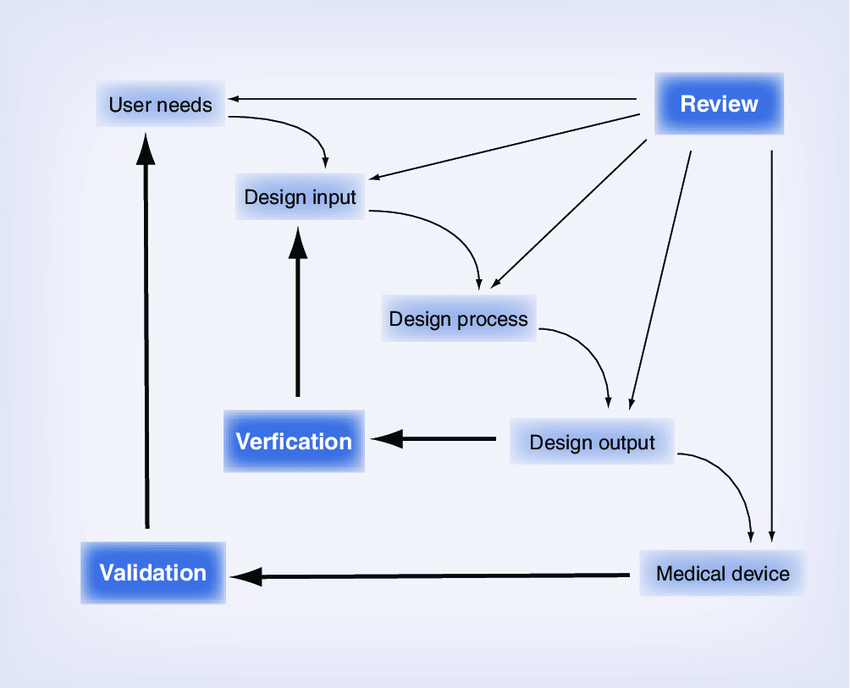
The FDA Waterfall provides a structured method to approach the design process. User needs are developed after customer discovery and needs identification. At the design input stage, design inputs translate the user needs into quantifiable design features of the device, which are often provided as a range and can be used to guide the design process. Once the design inputs have been selected, the design process is iteratively completed and the design outputs, also known as specifications, are developed. Design outputs are verified via testing to meet the design inputs originally developed. These design outputs directly guide the design of the medical device. Once the medical device is developed, validation testing is performed to ensure the device meets its original high level user needs. FDA design controls presentation and documents.