CERVICARE
A novel device to revolutionize cervical dilation and significantly enhance patient safety
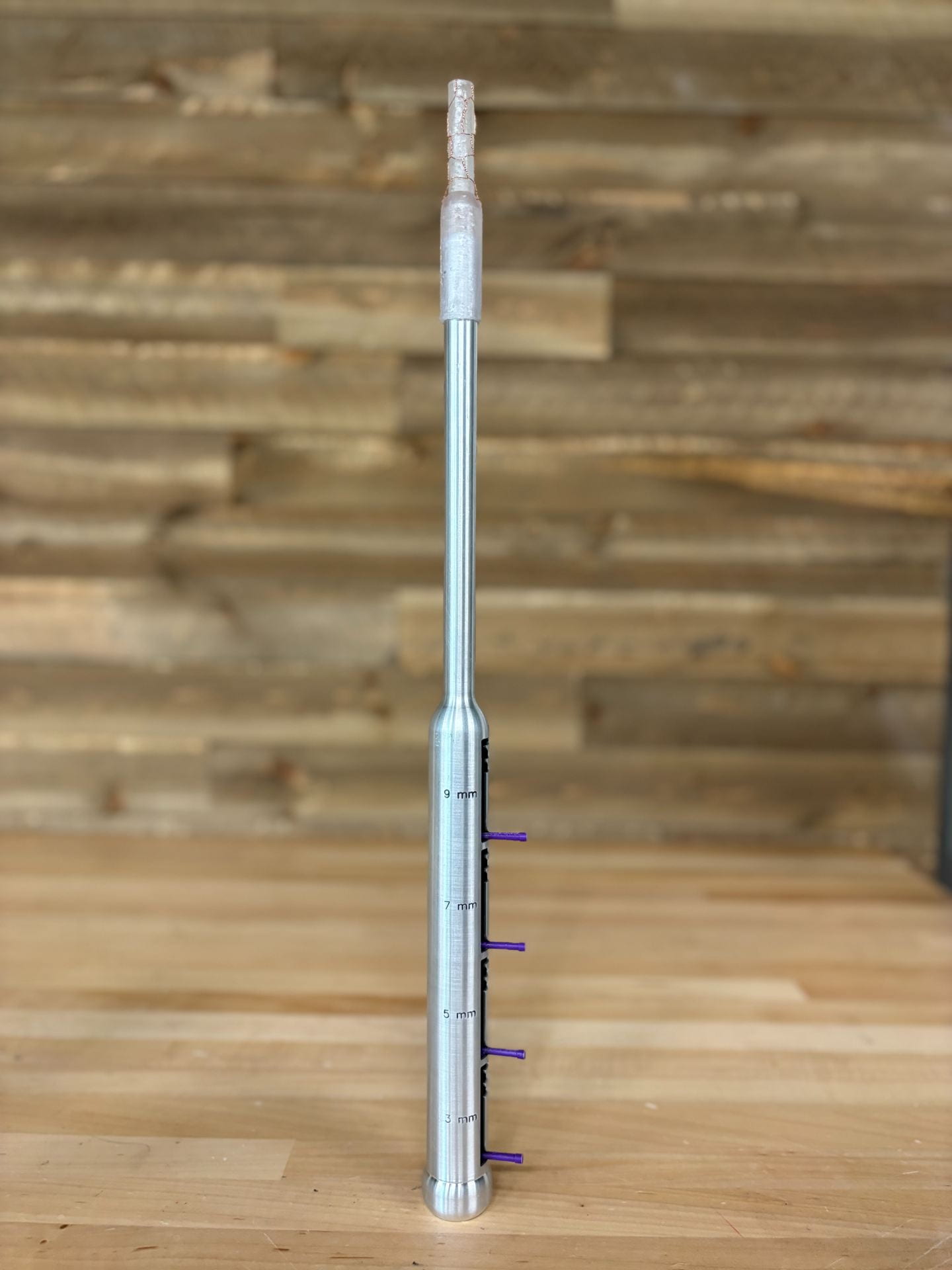
The CerviCare dilator features a series of telescoping sheaths encased in a flexible outer covering to revolutionize cervical dilation, enhance patient safety, and increase procedural efficiency. This device allows for complete cervical dilation with one insertion and is engineered so that previously neglected groups, such as individuals with stenotic cervixes, can also benefit.
Project Description:
Cervical dilation is the process of expanding the cervix using plastic or metal dilator rods of increasing diameters. Widening the cervix is required to allow surgical tools access to the uterus. However, using dilators can cause perforations of the uterus and surrounding organs, and can separately lead to the creation of false cavities in the cervical canal due to renavigation. Perforations can lead to life-threatening consequences, including small bowel obstruction, hemorrhage, sepsis, and death. False passageway creation occurs when the dilator enters an unintended space and is more common in patients that suffer from uterine abnormalities, such as retroversion, or cervical stenosis. Current cervical dilation methods have largely remained unchanged since the 1800s, exacerbating the need for an updated solution to these problems. Approximately 36 million cervical dilations occur within the United States annually for a variety of procedures, including cervical cancer diagnoses, endometrial ablations, and hysteroscopies. Hysteroscopy is the most frequently performed procedure that requires cervical dilation and approximately 50% of hysteroscopic complications (perforations, false passageway, and tears) are directly related to difficulty with cervical entry due to insufficient dilation. To solve this problem, our team has developed the CerviCare dilator. The device uses a telescopic method to achieve complete cervical dilation with just one initial insertion. Hollow sheaths of increasing diameters are slid over each other to sequentially dilate the cervix. All sheath advancement is controlled via a ratcheting mechanism in the handle and contained within a liquid silicone rubber outer covering. The outer covering reduces the risk of perforation by allowing for visibility under ultrasound through an embedded copper stent and a shock absorbent buffer at the distal tip. The outer covering can be detached post-dilation, serving as a channel for safe passage of surgical instruments. This directly eliminates the need for cervical renavigation.