Connect our team on LinkedIN!
Shayla, Ellington; Peyton, Holzworth; Abigail, Palmieri; Christina, Sun
Ecz-factor
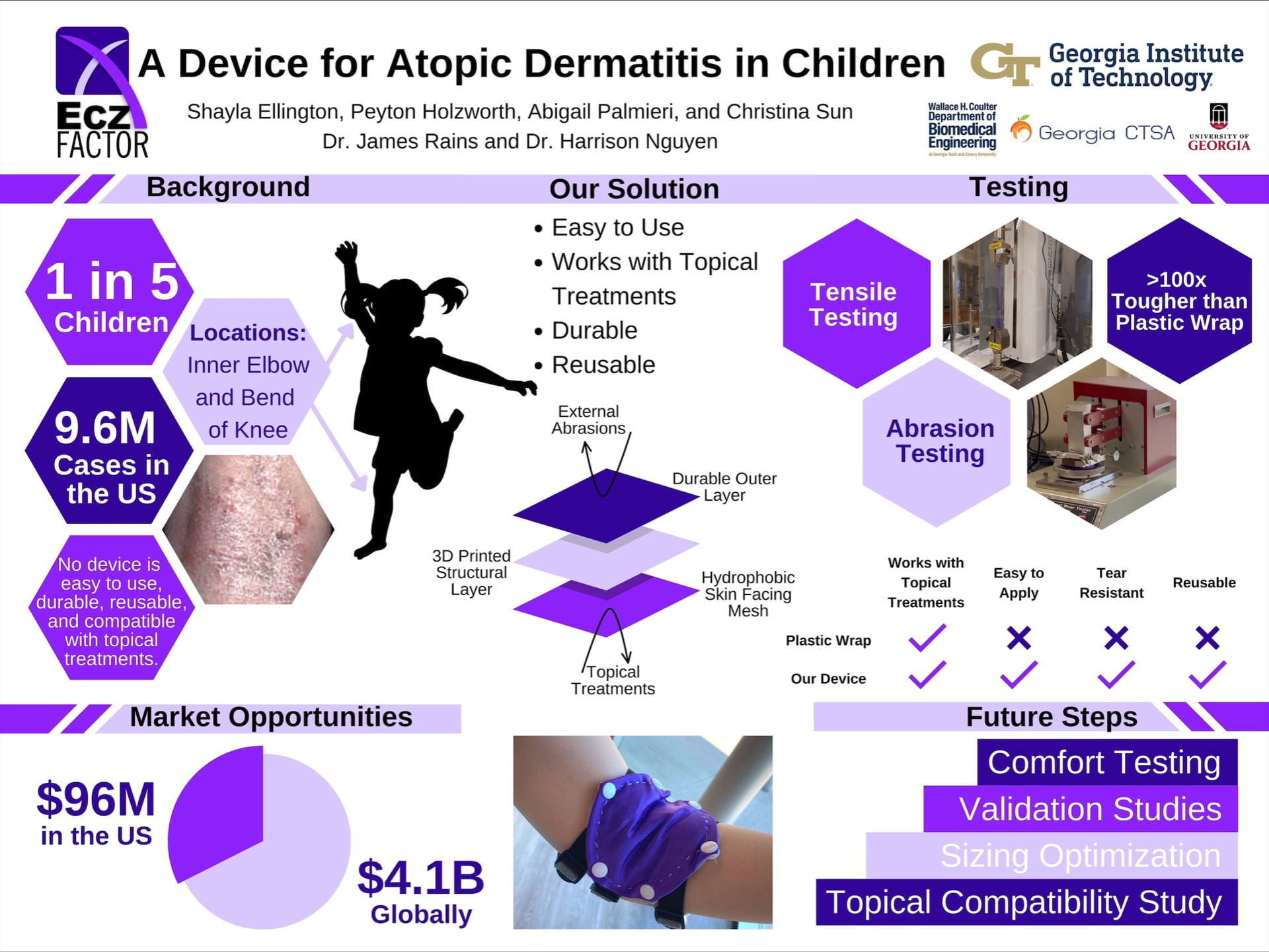
An At-Home Device for Atopic Dermatitis Relief
Ecz-Factor Prototype: The device has three layers. The purple material is a performance nylon and spandex outer layer with white snaps to connect between it and the skin-facing layer. The white layer is the skin-facing layer which consists of a hydrophobic mesh. A 3D printed structure on tulle is snapped between the outer and skin-facing layer. The black and gray sections on the straps are elastic and cotton, respectively. The straps are adjustable via ladder lock buckles. The black squares on the straps are the gel foam constructs for added friction to keep the device in place.