Get to know our team on LinkedIN:
Christina Draughon, Talley Motes, Delina Sheth, Courtney Smith, Jocelyn Vega
Epipals
Increase the adherence of epinephrine for those with allergies requiring them to have an epinephrine autoinjector accessible.
Project Description:
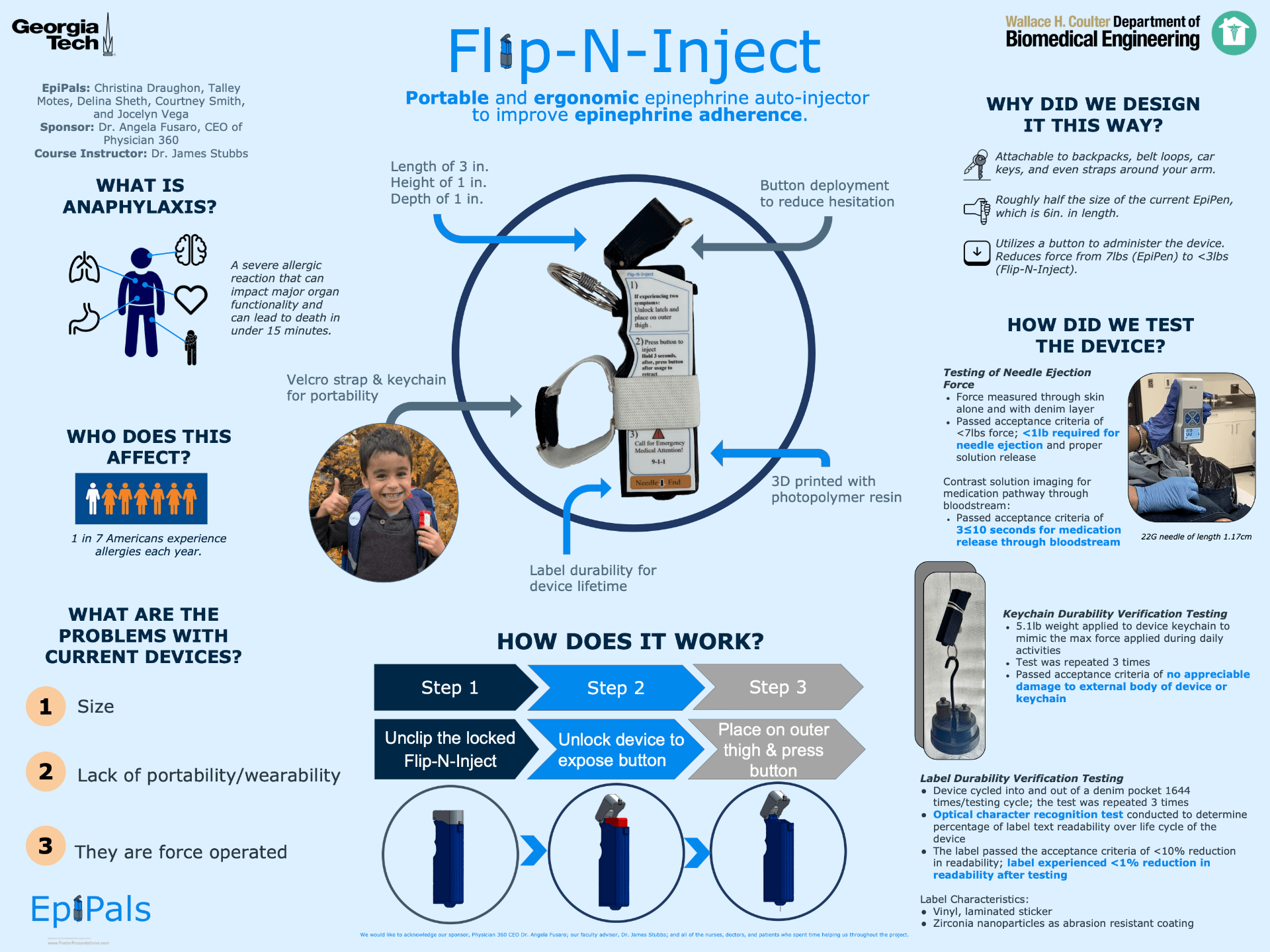
One in fifty Americans will experience an anaphylactic reaction in their lifetime. Anaphylaxis is a severe allergic reaction that can occur within seconds of exposure to an allergen and can become life-threatening. Epinephrine is the first line treatment for anaphylaxis, thus epinephrine autoinjectors are prescribed to those with severe allergies. They are meant to be carried on a daily basis, yet research has found that only 50% of patients carry their epinephrine autoinjectors regularly. From feedback compiled through user interviews, our team identified three main problems with the current marketed devices: size, portability, and the force required to administer the epinephrine. To address these issues, our team developed the Flip-N-Inject. The size hindrance of the current autoinjectors was eliminated by changing the internal mechanism of the device to accommodate a smaller and more user-friendly external design. The portability of the device was improved through incorporating a velcro strap and a durable keychain attachment with an easy-to-use clip for quick removal of the device. In terms of reducing hesitation to use the device, our team chose to incorporate a button mechanism to deploy the medication. We also improved the label on the device to include instructions on both when and how to inject the medication. Verification testing was run via failure testing on the keychain and durability testing on the label with the assistance of a robotic arm followed by an optical character recognition analysis. In addition, the penetration force of the needle and the solution release time was confirmed through cadaver testing. Research into the prior literature and patent landscape demonstrated freedom to operate. We estimate the serviceable market to be about $1.8 billion/year.